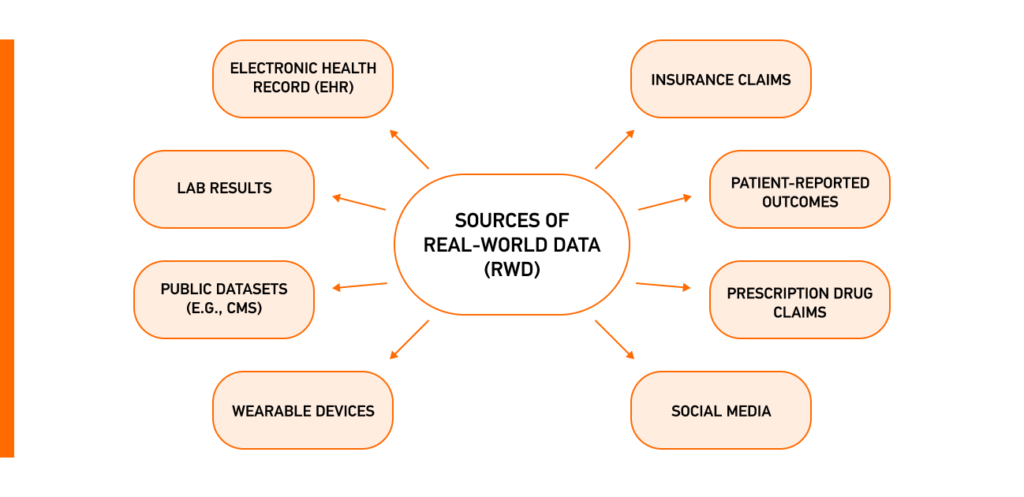
Real-world data (RWD) encompasses patient information sourced from doctors and a variety of outlets such as health records, insurance claims, and mobile devices. It captures real-life conditions, drawing different types of RWD, including:
- clinical data
- public health data
- patient-generated data
- cost data
This information is extracted from many sources, which presents a broader picture of patient needs.
Also, the insights from RWD empower patient services with real-world evidence (RWE), enhancing the development of medicines, treatments, and research. By understanding the basics of RWD, we pave the way to a future where healthcare professionals and patients can make more informed treatment decisions, transforming the landscape of healthcare delivery.
Another essential aspect of leveraging data for actionable insights in healthcare is ensuring the best security practices. This is why, when developing the data analysis platform for Zoadigm, we rigorously adhered to HIPAA guidelines to protect sensitive information and ensure compliance with laws and regulations.
In this article, we’ll explore how to increase patient empowerment and control over their health information through the efficient, ethical use of real-world data for healthcare, the advantages of this data, and strategies for overcoming its challenges.
Benefits of Real-World Data in Healthcare
- Timely Insights: RWD provides immediate insights into patient conditions and treatment results, keeping healthcare professionals up-to-date on changing situations and enabling prompt, informed decision-making.
- Diverse Data Sources: By including data from different sources, including electronic health records, wearable devices, and social media, RWD gives a thorough view of a patient’s health, helping healthcare professionals better understand their needs.
- Cost-Effectiveness: RWD leverages data from existing data sources to generate evidence required for trials, which is less costly than traditional clinical trials.
- Broad Patient Representation: RWD provides evidence that mirrors real-world demographics and experiences by including a more diverse patient population.
The use of real-world healthcare data for patients and healthcare professionals is pushing the industry toward a patient-centric and data-driven approach to care.
How Can Data Be Used in Patient Care? Use Cases
Use Case #1: Use of Real‐World Evidence to Drive Drug Development Strategies and Inform Clinical Trial Design
RWD contributes to a more efficient and patient-oriented healthcare paradigm by ensuring the immediacy of insights into the cost-effective utilization of existing data sources. However, healthcare data is always associated with ethical and privacy concerns, especially when extracting insights from sensitive data sources such as EHRs, which adds to real-world data challenges.
This use case displays how biopharmaceutical companies can inform internal decisions throughout the product development process by using RWD for the following tasks:
Guiding Pipeline and Portfolio Strategy: employing RWD to assess the commercial viability of drug development programs and identify patient subgroups of interest based on comprehensive RWD analysis.
Incorporating Novel Sources of RWD: integrating diverse types of data, such as genomic data, clinicogenomic data, medical imaging, radiomic data, and patient-derived xenograft data, that complement traditional sources like electronic health records for a more thorough analysis and understanding of real-world scenarios in healthcare.
Defining Clinical Development: using insights from RWD for shaping clinical trial eligibility criteria, optimizing trial populations, guiding endpoint selection and sample size estimation, and enhancing diversity among trial participants.
Using real-world data for healthcare research in biopharmaceutical companies throughout the product development cycle effectively addresses research questions related to patient populations, healthcare utilization, standard of care, and disease progression.
Use Case #2: Sharing Patient-Controlled Real-World Data Through the Application of the Theory of Commons
This study focuses on patient-controlled real-world data, aiming to propose a conceptual model that supports individual and population health while ensuring data privacy and control in chronic care management. It describes three arenas for collaboration and explains how real-world data can be used and shared across the following arenas while providing care for individuals with cystic fibrosis.
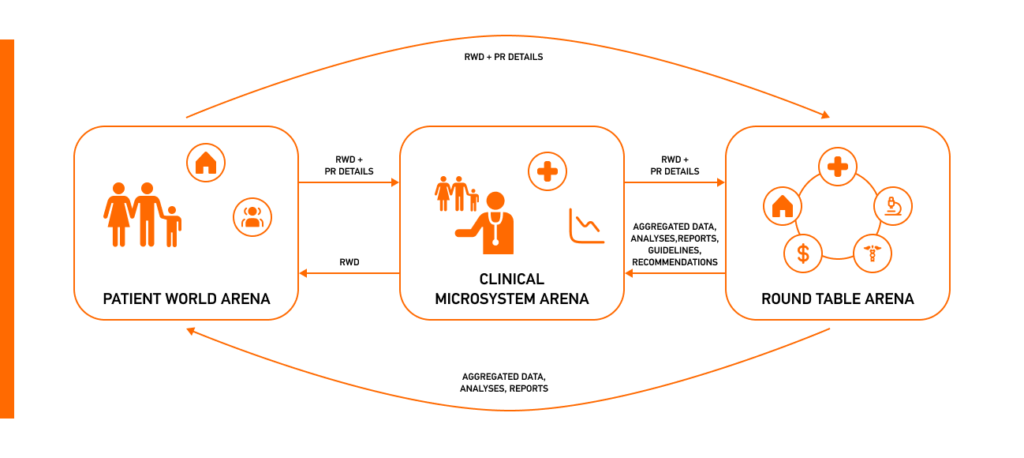
RWD for Patient World Arena: Patient-controlled real-world data captures nonphysical aspects of illness, problems, and behavior: patients and their trusted network contribute, extract, and manage data within this private domain. The sharing of patient-controlled real-world data is facilitated through health information exchanges, ensuring adherence to information standards, terminologies, and legal considerations.
RWD for Clinical Microsystem Arena: This arena is represented by cystic fibrosis care centers, in which teams engage with patients for quarterly check-ups and ensure a collaborative approach to healthcare. Healthcare professionals collect and store patient data and incorporate it through an export interface for further management and processing, which allows for gaining valuable insights.
RWD for Round Table Arena: This collaborative network includes various stakeholders: hospitals, authorities, and patients. This arena leverages RWD from the national quality registry to facilitate discussions, innovative healthcare approaches, and therapy implementation by modifying facilities, negotiating core data sets, and implementing new modules for therapy follow-up.
This research shows that by specifying property rights for using RWD for different healthcare actors, we ensure patient control over health information, promote the collaborative use of RWD, and gain valuable insights that support high-quality healthcare. The proposed model can help leverage RWD to provide insights applicable to diverse chronic care settings and ensure patient-centric, data-driven healthcare practices.
RWD is a base for a comprehensive analysis of patient data that incorporates diverse data sources, optimizes trial parameters, and supports chronic care management. From these use cases, it becomes clear that the effectiveness and ethics of RWD use depend on the data governance model.
Let’s define the most critical components of data governance that can help leverage RWD to ensure informed and collaborative healthcare practices.
Real-World Data Governance for Safe Access to Patient Information
Data Security and Regulatory Compliance
The requirements for regulatory compliance in healthcare make stakeholders stick to robust data security measures to ensure transparent communication between systems. Given that RWD involves diverse systems and data sources that store sensitive patient data (EHRs, clinical data registries, etc.), ensuring compliance with data protection regulations is necessary.
While constructing an FHIR semantic layer analysis platform that employs data from various electronic health records and additional sources, our team strictly followed HIPAA’s guidelines to uphold the privacy and security of data throughout the project.
Data anonymization techniques are another critical aspect that helps maintain patient data privacy. For a national eHealth system, Edenlab’s team used the pseudonymization mechanism recommended by the European GDPR. This approach separates medical data from personal data using an intruder that cannot be linked to a specific patient. Pseudonymization is especially useful when using RWD, which involves chronic health conditions data and other sensitive information that, if leaked, can lead to dangerous consequences for a patient.
Data Quality
Data quality in healthcare is measured through key metrics: validity, reliability, cohesiveness, uniqueness, and timeliness. Neglecting these aspects can lead to errors in healthcare data that will lower the efficiency of real-world healthcare data analysis and its further use for patient care. However, there are several ways to ensure the highest quality healthcare data, including:
- Data Standardization: Leveraging a common data model allows for representing different healthcare concepts while ensuring the accuracy of the data when it is exchanged and entered. In our article on FHIR vs. HL7, we shared different versions of the most popular healthcare data standards and their use cases.
Given the growing popularity of FHIR and its active implementation within various national healthcare systems, building an FHIR-first system can help to connect with multiple data sources and leverage this data for complex tasks like population health analysis and reporting.
- Data Cleaning: Another way to ensure data quality is by minimizing record errors and duplicates. Our team developed the master patient index for a national healthcare system to improve data quality through a comprehensive duplicate identification model.
Incorporating principles from fintech scoring and deduplication processes enabled the implementation of a scoring model using supervised machine learning. The process involves online and offline deduplication, including checks for potential duplicate identification as a new patient profile is created, and a search for duplicates is performed offline for additional layers of scrutiny.
Patient Engagement and Consent
Consent is important when dealing with RWD or any other personal information. GDPR prohibits processing personal data unless expressly allowed by law or with the explicit consent of the data subject.
Hence, to drive patient empowerment, establishing a robust content management framework is necessary: it enables patients to provide informed consent for data sharing. Consent management helps stakeholders comply with data protection requirements and ensures the effective and trustworthy use of data within healthcare, fostering patient trust in the system.
FHIR for the Effective Use of RWD in Healthcare
By leveraging the FHIR standard, stakeholders can cover all the aspects of RWD data governance at once. The Vulcan project develops methods for using the HL7 FHIR standard to retrieve relevant RWD from EHR systems.
The Vulcan Project developed the FHIR Implementation Guide, which defines a minimal set of clinical research FHIR resources and EHR elements that can be interoperable and consistently used for clinical research purposes. Solutions like the Kodjin FHIR server can be instrumental in implementing these standards at scale.
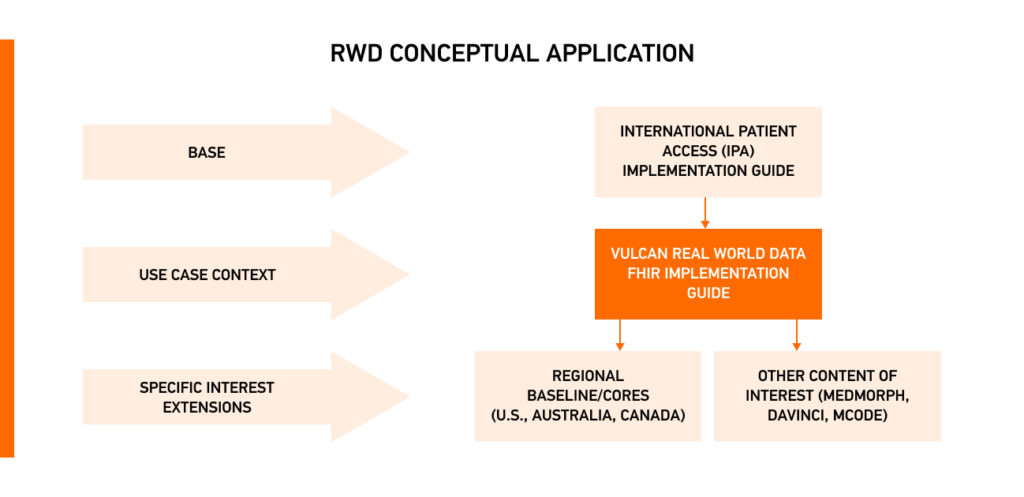
This guide relies on the International Patient Access (IPA) project for foundational data to build FHIR profiles. It outlines FHIR components to refine standard resources by identifying research questions and creating corresponding profiles. This helps establish future standards like US Core, Australia Core, and Japan Core, with mappings from projects like FHIR to CDISC and FHIR to OMOP to ensure the effective use of RWD for research.
Let Us Help You Leverage Real-World Data
Our team boasts a proven track record in custom development services, having successfully navigated the complexities of high-load and national-scale projects with the FHIR standard. The team of experts at Edenlab is ready to design a solution that matches the specific needs of your project and allows for leveraging healthcare data from various data sources while ensuring the highest level of interoperability.
In the Kodjin Interoperability Suite, you’ll find the essential tools for custom profiling, data normalization, and regulatory compliance. Do not hesitate to consult our professionals to discover how our solutions, strategies, and extensive healthcare domain knowledge can help you achieve your project’s goals.






